A research paper titled Amino Acids Trapped Inside C₁₀₀: A Computational Study, co-authored by Dr Surajit Kayal, Assistant Professor, Chemistry, SIAS, and Dr Brijesh Kumar Mishra, Associate Professor, Chemistry, SIAS, has recently been published in the scientific journal ChemPhysChem.
Abstract
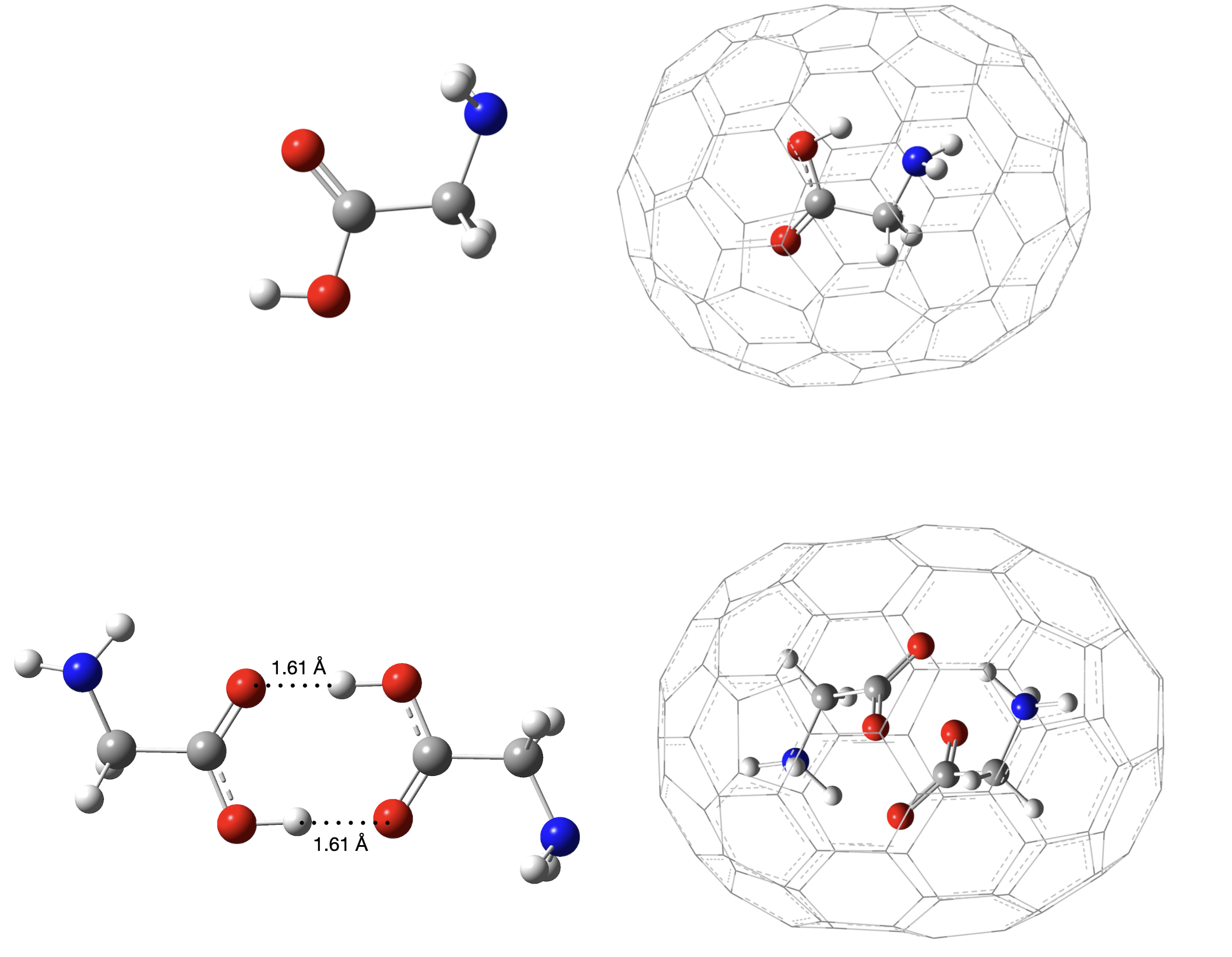
The feasibility of the C₁₀₀ fullerene as a nanocontainer for glycine, alanine, and serine has been investigated using density functional theory (B3LYP-D3), second-order Møller–Plesset perturbation theory, and the domain-based local pair natural orbital–coupled cluster singles doubles and perturbative triples (DLPNO-CCSD(T)) method. The interaction energies for glycine@C₁₀₀, alanine@C₁₀₀, and serine@C₁₀₀ are calculated to be −47.8, −45.5, and −43.8 kcal mol−1, respectively, for their most stable conformers at the DLPNO-CCSD(T) level, indicating favourable host–guest interactions. Furthermore, encapsulation leads to substantial stabilisation of both the intramolecular hydrogen-bonded and non-hydrogen-bonded conformers of the amino acids. Vibrational frequency analysis shows a blueshift for most vibrational modes, indicative of restricted motion due to the confined space. However, the OH-stretch mode, especially for the intramolecular hydrogen-bonded conformers, exhibits a large redshift upon encapsulation, suggesting a strengthening of the hydrogen bond due to confinement. Dipole moment calculations reveal a significant reduction after encapsulation, indicating effective screening of the dipole by the C₁₀₀ cage. ¹H NMR chemical shift calculations show a large downfield shift, consistent with deshielding effects experienced by the encapsulated molecules due to the unique electronic environment within the fullerene cavity.

